Highlights
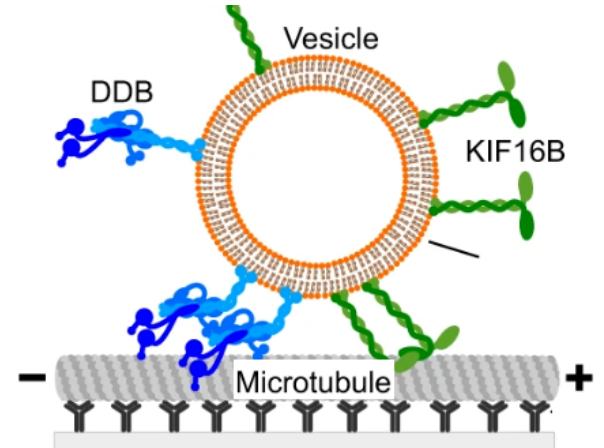
Vesicles driven by dynein and kinesin exhibit directional reversals without regulators
Intracellular vesicular transport along cytoskeletal filaments ensures targeted cargo delivery. Such transport is rarely unidirectional but rather bidirectional, with frequent directional reversals owing to the simultaneous presence of opposite-polarity motors. So far, it has been unclear whether such complex motility pattern results from the sole mechanical interplay between opposite-polarity motors or requires regulators.
In our recent publication in Nature Communications, we - together with the group of Stefan Diez (TU Dresden) - demonstrate that a minimal system, comprising purified Dynein-Dynactin-BICD2 (DDB) and kinesin-3 (KIF16B) attached to large unilamellar vesicles, faithfully reproduces in vivo cargo motility, including runs, pauses, and reversals. Remarkably, opposing motors do not affect vesicle velocity during runs. Our computational model reveals that the engagement of a small number of motors is pivotal for transitioning between runs and pauses. Taken together, our results suggest that motors bound to vesicular cargo transiently engage in a tug-of-war during pauses. Subsequently, stochastic motor attachment and detachment events can lead to directional reversals without the need for regulators.
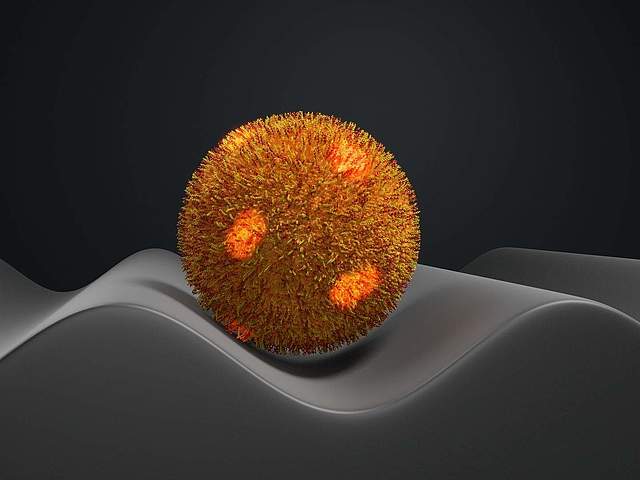
Adhesiveness of Staphylococcus aureus cells is unevenly distributed across the cell surface
Staphylococcus aureus, a dreaded hospital germ, can cause serious infections. One remarkable characteristic of this bacterium is its extraordinary adhesive ability. A team from the Physics and Medicine departments at Saarland University has now discovered that the germ adheres better to some parts of the envelope than to others. Their study, which has now been published, could be the starting point for more effective bacteria-repellent surfaces.
Press release of Saarland University of 17.10.2023 (in German)
Publication:
C. Spengler, E. Maikranz, B. Glatz, M.A. Klatt, H. Heintz, M. Bischoff, L. Santen, A. Fery and K. Jacobs: “The adhesion capability of Staphylococcus aureus cells is heterogeneously distributed over the cell envelope”, Soft Matter, 2023, Advance Article.
