RESEARCH
OVERVIEW

In our group, we are focussed on all kinds of halogenated compounds, their properties and how to synthesize them in a mild and selective manner. We therefore set our focus on developing biomimetic synthesis methods.
Although the halogenation of organic molecules is one of the most widespread techniques for the functionalization of substrates, efficient catalytic methods for the selective installation of halogen atoms are rare. Our research program therefore addresses the long-standing problem of catalytic halogenation. Nature has developed different strategies to catalyze these types of reactions with high chemo-, regio, and stereospecificity. By exploring and emulating Nature’s concept, new catalysts are elaborated, which allow for the development of mild, generally applicable, and selective catalytic methods for the formation of carbon-halogen bonds, thus adding a particularly advantageous tool to the arsenal of methods in modern synthesis. With the application of these catalysts, novel compound classes with unique structures and potent biological activities are easily accessed, expanding the boundaries of chemical space, which will contribute to the discovery of new lead compounds and has the potential for the development of new drugs and theranostic biomaterials.
CONTACT
Prof. Dr. Tanja Gulder
Head of the group
Campus C4 2, Room 0.04
66123 Saarbrücken
Phone: +49 341 97-36540
PROJECTS
Mild and Selective, Biomimetic Halogenations
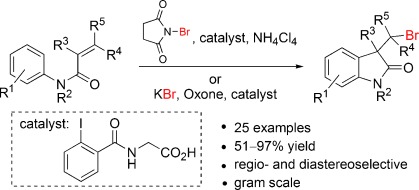
Many of our biomimetic halogenation efforts employ iodanes as mild halogenating agents. Our first efforts in this direction started in 2012 when we published our iodine(III)-catalyzed bromocarbocyclization synthesis of 3,3-disubstituted oxoindoles.[22]
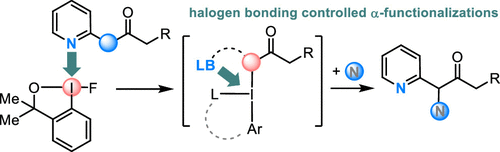
Building upon those initial findings, we were also able to employ iodanes to synthesize α,α-dialkylated α-hydroxy carboxylamides[32], fluoro benzoxazepines[33] and α-hydroxy-β-amino acids[36]. We were also able to use fluoro-iodanes to facilitate α-functionalization of ketones via a nitrogen directed oxidative umpolung[48]. An overview on our efforts and the development of the described methods is given here [54].
In further efforts we were able to use F-Iodane to trigger semipinacol rearrangement in addition to the known fluorination and aryl migration cascade reaction of styrene derivatives. This method can be used for the synthesis of various cyclopentanones bearing tertiary C,F-carbon centers adjacent to a ketone group[58].
[22] D. C. Fabry, M. Stodulski, S. Hoerner, T. Gulder; Chem. Eur. J. 2012, 18, 10834-10838. FULLTEXT
[32] A. Ulmer, M. Stodulski, S. V. Kohlhepp, C. Patzelt, A. Pöthig, W. Bettray, T. Gulder; Chem. Eur. J. 2015, 21, 1444-1448. FULLTEXT
[33] A. Ulmer, C. Brunner, A. M. Arnold, A. Pöthig, T. Gulder; Chem. Eur. J. 2016, 22, 3660-3664. FULLTEXT
[36] C. Patzelt, A. Pöthig, T. Gulder; Org. Lett. 2016, 16, 3466-3469. FULLTEXT
[48] G. M. Kiefl; T. Gulder; J. Am. Chem. Soc. 2020; 142,20577–20582. FULLTEXT
[54] M. Kretzschmar; T. Gulder; Synlett 2022, 34, 405 - 413. FULLTEXT
[58] P. Zhao; W. Wang; T. Gulder; Org. Lett. 2023; 25; 6560-6565. FULLTEXT
Supramolecular Assemblies in fluorinated Alcohols
During our studies, we became increasingly aware of the unique properties of flourinated alcohols like HFIP (Hexafluorisopropanol), TFE (2,2,2-Trifluorethanol), and PFTB (Perfluoro-tert-butanol). We were especially pleased, when we observed a very distinguished improvement when employing HFIP as the solvent for our biomimetic haliranium-induced polyene cyclizations, which are traditionally quite difficult to achieve.[42] We ascribed this effect to the HFIPs ability to form microheterogenous, supramolecular assemblies which we proposed to be able to pre-arrange the substrate of the reaction in a favourable conformation.
Inspired by other supramolecular approaches, we explored polyene cyclizations using dynamic yet structurally defined macromolecular assemblies to extend artificial enzyme catalysis. By forming catalytically active Lewis acid–Lewis base complexes from fluorinated alcohols and ammonium or pyridinium salts, we enable efficient, stereocontrolled cyclizations of diverse alkenes. This method is highly functional group tolerant, operates under mild conditions, and relies on inexpensive, readily available materials.[55]

We introduced a selective and versatile approach using HFIP–chloroiodane networks to mimic terpene cyclases for chlorination-induced polyene cyclization, which have been underexplored ntil yet. This method enables highly selective transformations of various alkenes, including complex terpenes and terpenoid frameworks.[56]
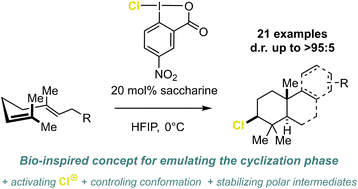
We are now focussed on further investigating and characterizing these interactions and utilizing supramolecular assemblies in other reactions, such as photochemical transformations etc.
[42] A. M. Arnold, A. Pöthig, M. Drees, T. Gulder; J. Am. Chem. Soc. 2018,140, 4344–4353. FULLTEXT
[55] A.M. Arnold; P. Dullinger; A. Biswas; C. Jandl; D. Horinek; T. Gulder; Nat. Commun. 2023, 14, 813. FULLTEXT
[56] J. Binder; A. Biswas; T. Gulder; Chem. Sci. 2023, 14, 3907-3912. FULLTEXT
Halogenated Natural Products and Halogenating Enzymes
As we are trying to mimick nature by establishing biomimetic halogenation reactions, we are interested in closely investigating how exactly nature performs these challenging and quite rare transformations. One type of halogenating enzymes we are especially interested in are Vanadium-dependent haloperoxidases (VHPO). We were able to characterize a cyanobacterial haloperoxidase (AmVHPO) and evaluate its biocatalytic halogenation potential.[38]
From there, we were also able to establish a photobiocatalytic halogenations by using atom‐economic electron donors for the haloperoxidase.[44]
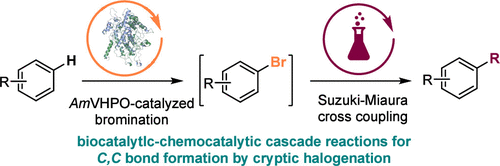
In further investigations and inspired by natural halogenation in C–C bond formation, we expanded the scope of biocatalytic bromination with transition-metal-catalyzed cross-coupling. Utilizing the cyanobacterial AmVHPO, a robust bromination-arylation cascade was developed, with enzyme immobilization improving biocatalysis-chemocatalysis compatibility.[65]
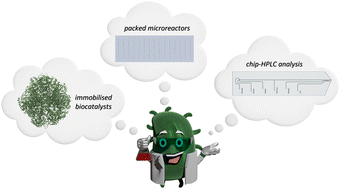
The highly potential use of VHPOs of Curvularia inaequalis (CiVHPO) could be demonstrated in the development of a novel microfluidic screening setup with real-time analytics for studying reactions with immobilized biocatalysts. By integrating microreactor technology, automated multi-reactor screening, and online LC/MS analysis, we enable efficient reaction monitoring and optimization. As a proof of concept, we optimized an aromatic bromination using immobilized CiVHPO, demonstrating the system’s potential for data-rich, resource-efficient biocatalysis.[62]
We are also interested in unraveling the mechanism behind substrate specifity and halogen activation of VHPOs. Structural analysis reveals a newly formed substrate-binding site that enhances substrate residence time and stabilizes intermediates, providing crucial insights into VHPO mechanism and expanding their potential for diverse chemical applications.[66]

Our current efforts are focussed on achieving the catalytic, electrochemical iodane-formations as well as delving further into the development of new, enantioselective halogenation methods.
[38] A. Frank, C. J. Seel, M. Groll, T. Gulder; ChemBioChem 2016, 17, 2028-2032. FULLTEXT
[44] C. J. Seel, A. Králík, M. Hacker, A. Frank, B. Koenig, T. Gulder; ChemCatChem 2018, 10, 3960-3963. FULLTEXT
[62] S. Schmidt; H. Westphal; S. Lama; M. Polack; C. Weise; T. Oestereich; R. Warias; T. Gulder; D. Belder; React. Chem. Eng. 2024, 9, 1739-1750. FULLTEXT
[65] Q. Zhao; R. Zhang; J. Döbber; T. Gulder; Org. Lett. 2024, 27, 1, 159–164. FULLTEXT
[66] P. Zeides; K. Bellmann-Sickert; R. Zhang; C. J. Seel; V. Most; C. Schoeder; M. Groll; T. Gulder; Nat Commun 2025, 16, 20381. FULLTEXT
Electrochemical Deuteration
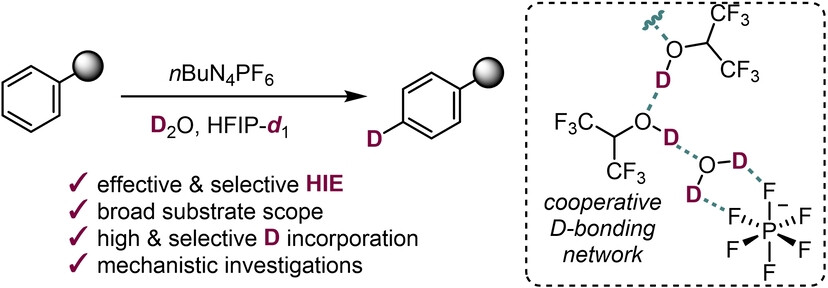
We are interested in development of methods for hydrogen isotope exchange (HIE) in arenes using PF6− in deuterated HFIP and D2O enables high yields and excellent deuterium incorporation under mild conditions. An extended H-bonding network activates the inert P−F bond, facilitating HIE in phenols, anisoles, anilines, and heteroaromatics.
We are currently interested in the research on sustainable electrochemical methods for the selective incorporation of deuterium into organic molecules.
[64] Y. Ni; J. Lebelt; M. Barp; F. Kreuter; H. Buttkus; J. Jin; M. Kretzschmar; R. Tonner-Zech; K. Asmis; T. Gulder; Angew. Chem. Int. Ed. 2024, e202417889. FULLTEXT