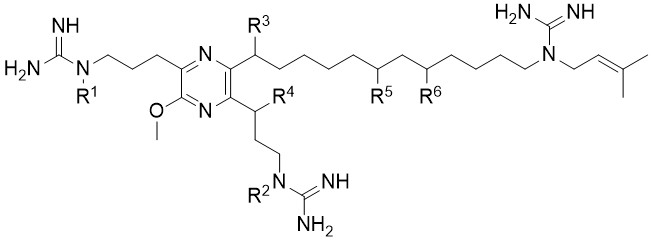
Crossiguanipyrazines
The discovery of several crossiguanipyrazines, a rare family of potent anti-TB alkylpyrazines isolated from the underexplored actinobacterium Crossiellacryophila DSM 44230 is described. Crossiguanipyrazines (CGPs) feature an unusual 3-methoxyl-2,5,6-trialkylpyrazine scaffold with varying degrees of hydroxylation and N-prenylation. Stable isotope-labeling experiments indicated a unique biosynthetic origin involving arginine and acetate, while inhibition studies with a cytochrome P450 inhibitor suppressed hydroxylation, implicating P450 enzymes in this transformation. The structure of crossiguanipyrazine I is validated by total synthesis.
- H. Zhao, E. Bickel, J. Liu, X. Yang, M. Dal Molin, H. Sui, X. Bian, J. Rybniker, U. Kazmaier, C. Fu, "Discovery and Total Synthesis of Crossiguanipyrazines with Potent Activity against Mycobacterium tuberculosis", Angew. Chem. Int. Ed. 2025, e13977. DOI: 10.1002/anie.202513977