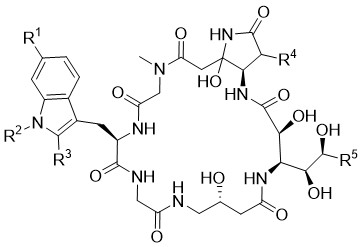
Microsclerodermin
From 1994 until 2000, the Faulkner Group isolated several microsclerodermins from marine sponges of the Microscleroderma and Theonella species. Further derivatives have been isolated later on by the groups of Li and Matsunaga from other sponges. In 2013, the Müller group described some more microsclerodermins from three different genera of terrestrial Myxobacteria, which underpins the theory that these "sponge metabolites" might, in fact, originate from microbial symbionts genetically related with myxobacteria. Most members of the microsclerodermin family including the dehydrated derivatives, show potent antifungal activity against Candida albicans as well as activities towards various cancer cell lines.
- C. A. Bewley, C. Debitus, D. J. Faulkner, J. Am. Chem. Soc. 1994, 116, 7631–7636.
- A. Qureshi, P. L. Colin, D. J. Faulkner, Tetrahedron 2000, 56, 3679–3685;
- X.-C. Li, X. Zhang, M. Jacob, R. Ranga Rao, Y. Wang, A. Agarwal, D. Newman, I. Khan, A. Clark, Res. Reports Med. Chem. 2013, 3, 9.
- T. Hoffmann, S. Müller, S. Nadmid, R. Garcia, R. Müller, J. Am. Chem. Soc. 2013, 135, 16904–16911.
- T. Tian, K. Takada, Y. Ise, S. Ohtsuka, S. Okada, S. Matsunaga, Tetrahedron 2020, 76, 130997.
Starting from L-xylose and D-arabinose, six different cyclic microscleroderma derivatives were successfully obtained. Key steps of the syntheses are, on the one hand, Sakurai allylations, whose stereochemical course depends on the Lewis acid used, and on the other hand, photochemical Wolff rearrangements in the presence of complex aminofuranosides. Finally, aromatic side chains were introduced via cross metathesis.
- K. Bauer, U. Kazmaier, "Stereoselective Syntheses of Cyclic Microsclerodermin Derivatives", Chem. Eur. J. 2025, 31, e02459. DOI: 10.1002/chem.202502459