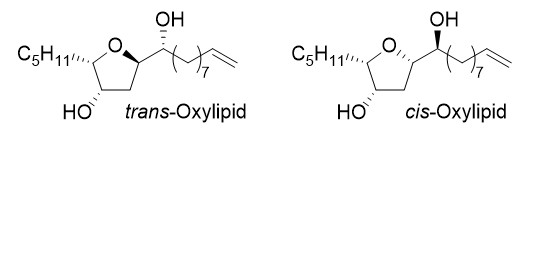
Oxylipide
Chemical analysis of N. anomala collected off rock platforms along the southern coast of Australia yielded cis-Oxylipid, a cis-dihydroxytetrahydrofuran, whose the structure was assigned by spectroscopic analysis, chemical derivatization and biomimetic synthesis. Tetrahydrofurans from Notheia anomala are reported for the first time as potent and selective inhibitors of the larval development of parasitic nematodes.
- R. Warren, R. Wells, J. Blount, Aust. J. Chem. 1980, 33, 891–898.
- R. J. Capon, R. A Barrow, S. Rochfort, M. Jobling, C. Skene, E. Lacey, J. H Gill, T. Friedel, D. Wadsworth, Tetrahedron 1998, 54, 2227–2242.
Deprotonated trimethylsilylethanol can be used as a nucleophile in Matteson homologations. This O-protection group is stable under the usual reaction conditions of the Matteson reaction, but after two further homologation steps it is automatically cleaved off and cycloetherification can take place, giving rise to substituted tetrahydrofurans in a highly stereoselective fashion. This elegant protocol was used in the synthesis of various oxylipids.
- M. Tost, U. Kazmaier, "Stereoselective Syntheses of Highly Substituted Tetrahydrofurans based on Matteson Homologations”, Chem Eur. J. 2025, 31, 202500560. DOI: 10.1002/chem.202500560
- M. Tost, U. Kazmaier, "Synthesis of Oxylipids via a Boronic Ester Cycloetherification Approach”, Org. Chem. Front. 2025, 12, 3288–3292. DOI: 10.1039/d5qo00213c