Research

Research Topics
1. Structure/function analysis of the Sec61 channel beta subunit (Sbh1 in yeast)
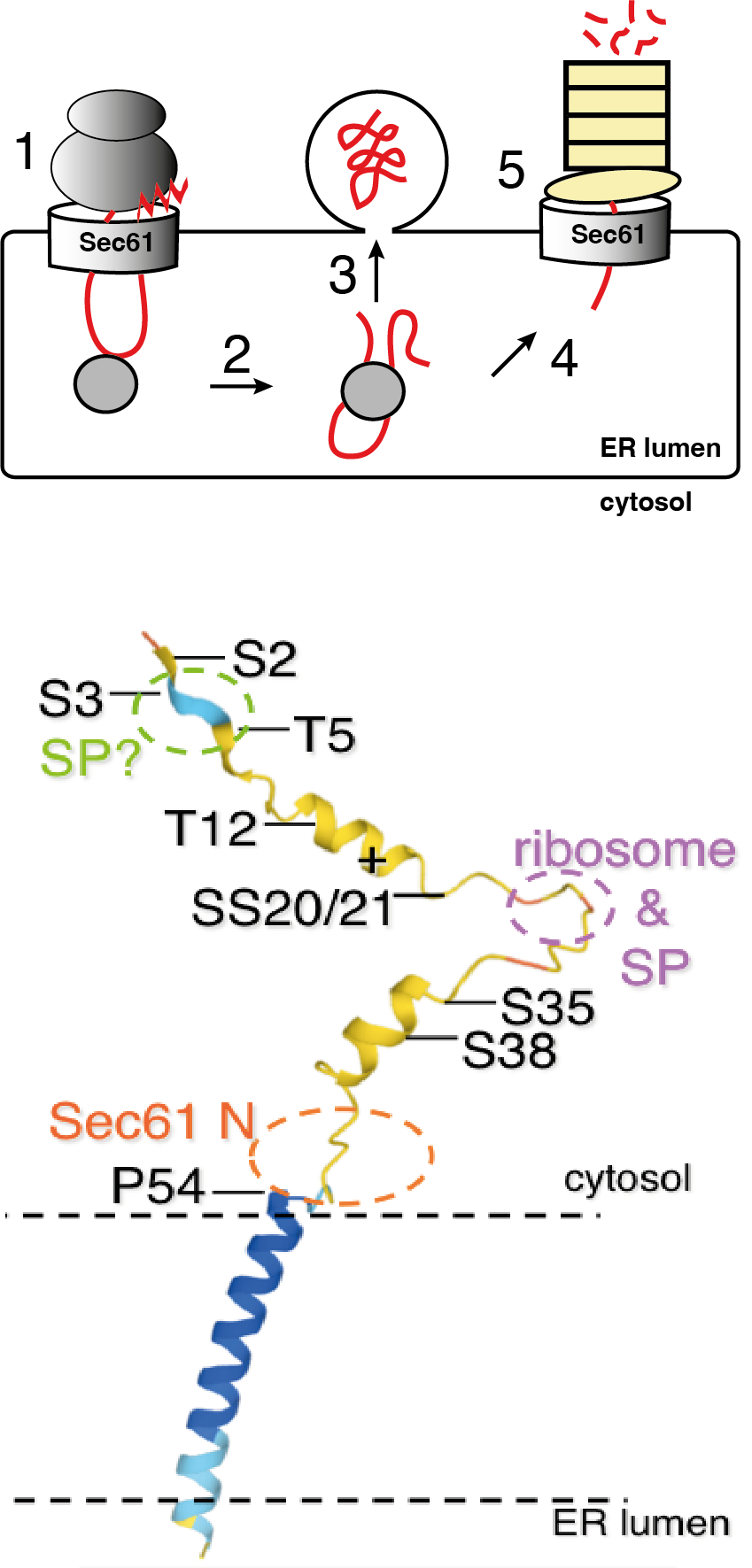
The Sec61 channel consists of 3 proteins, Sec61alpha, Sec61beta, Sec61gamma in mammals (Sec61, Sbh1, Sss1 in yeast). Sec61beta/Sbh1 is the only non-essential channel subunit, but is critical for infection by major plant and human pathogens. It is peripherally associated with the channel and consists of an intrinsically unstructured N-terminal, cytosolic domain which is poorly conserved and of varying length, followed by about 15 amino acids that are conserved and structured, and a single conserved transmembrane helix. The intrinsically unstructured part contains multiple (in yeast 8), partially conserved phosphorylation sites whose function is currently unknown.
Using a combination of site-directed mutagenesis, yeast genetics, and biochemical techniques and in collaboration with colleagues in Bioinformatics and Chemistry we are exploring the contribution of Sbh1 and its specific structural elements to protein import into the ER.
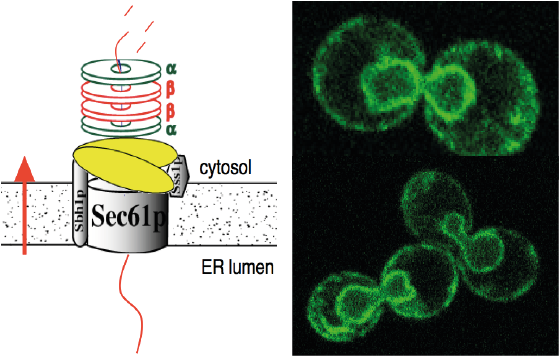
2. Roles of the Sec61 channel and the proteasome 19S particle in ERAD
We have shown that cytosolic proteasomes can bind to the Sec61 channel via their 19S regulatory particles and that this interaction is critical for the degradation of a soluble misfolded secretory protein. As our experiments were based mainly on ERAD reconstituted in a cell-free assay system we are now investigating the interaction of proteasomes with Sec61 channels in intact cells. The contribution of Sec61 to ERAD of misfolded membrane proteins has been called into question. We are therefore also exploring the contribution of the Sec61 channel to the degradation of misfolded membrane proteins.
Publications
Santiago-Tirado FH, Hurtaux T, Geddes-McAlister J, Nguyen D, Helms V, Doering TL, Römisch K. (2023). The ER Protein Translocation Channel Subunit Sbh1 Controls Virulence of Cryptococcus neoformans. mBio. 14(1):e0338422. doi: 10.1128/mbio.03384-22. Epub 2023 Feb 7.
Barbieri G, Simon J, Lupusella CR, Pereira F, Elia F, Meyer H, Schuldiner M, Hanes SD, Nguyen D, Helms V, Römisch K. (2023). Sec61 channel subunit Sbh1/Sec61β promotes ER translocation of proteins with suboptimal targeting sequences and is fine-tuned by phosphorylation. J Biol Chem 2023 299(3):102895. doi: 10.1016/j.jbc.2023.102895.
Bhadra P, Yadhanapudi L, Römisch K, Helms V (2021). How does the presence/absence of Sec63 affect Sec61 conformations? PLoS Computational Biology 17(3):e1008855.
Elia F, Yadhanapudi L, Tretter T, Römisch K (2019). The N-terminus of Sec61p plays key roles in ER protein import and ERAD. PLoS ONE 14(4):e0215950.
Pereira F, Rettel M, Stein F, Savitski MM, Collinson I, Römisch K (2019). Effect of Sec61 interaction with Mpd1 on endoplasmic reticulum-associated degradation. PLoS ONE 14(1):e0211180.
Kaiser ML & Römisch K (2015). Proteasome 19S RP binding to the Sec61 channel plays a key role in ERAD. PLoS ONE 10(2): e0117260.
Servas C & Römisch K (2013). The Sec63p J-domain is required for ER-associated degradation of soluble proteins in yeast. PLoS ONE 8(12): e82058.
Tretter T, Pereira FP, Kalies KU, Allan S, Helms V, Römisch K (2013). ERAD & protein translocation defects in a sec61mutant lacking ER-lumenal loop 7. BMC Cell Biol 14:56.
Harty C & Römisch K (2013). Analysis of Sec61p and Ssh1p interactions in the ER membrane using the split-ubiquitin system. BMC Cell Biol. 14:14.
Soromani C, Zeng N, Hollemeyer K, Heinzle E, Klein MC, Tretter T, Seaman MNJ, Römisch K (2012). N-acetylation and phosphorylation of Sec complex subunits in the ER membrane. BMC Cell Biol. 13:34.
Miranda E, McLeod I, Davies MJ, Perez J, Römisch K, Crowther DC, Lomas D (2008). The intracellular accumulation of mutant neuroserpin polymers explains the severity of the dementia FENIB. Hum Mol Gen 17, 1527-39.
Feng DJ, Zhao X, Soromani C, Toikkanen J, Römisch K, Keränen S, Jäntti J (2007). The trans-membrane domain is sufficient for Sbh1p function, its association with the Sec61 complex and its interaction with Rtn1p. J Biol Chem 282, 30618-28.
Ng W, Sergeyenko T, Zeng N, Brown JD, Römisch K (2007). Characterization of the interaction between the proteasome and the Sec61 channel at the ER membrane. J Cell Sci 120, 682-91.
Römisch K, High S, Miller FW, Dobberstein B (2006). Human autoantibodies against the 54 kDa protein of signal recognition particle block release of the nascent chain at the ER membrane. Arthritis Res & Therapy 8, R39.Kalies
KU, Alan S, Sergeyenko T, Kröger H, Römisch K (2005). The protein translocation channel binds proteasomes to the endoplasmic reticulum. EMBO J 24, 2284-93.
Miranda E, Römisch K, Lomas DA (2004). Mutants of neuroserpin that cause dementia accumulate as polymers within the endoplasmic reticulum and are degraded by proteasomes. J Biol Chem 279, 28283-91.
Lee RJ, Liu C, Harty C, McCracken AA, Römisch K, DeMartino GN, Thomas PJ, Brodsky JL (2004). The 19S (PA700) cap of the 26S proteasome is sufficient to retro-translocate and deliver a soluble polypeptide for ER-associated degradation (ERAD). EMBO J 23, 2206-15.
Scheper W, Thaminy S, Kais S, Stagljar I, Römisch K (2003). Coordination of N-glycosylation and protein translocation across the ER membrane by Sss1 protein. J Biol Chem 278, 37998-38003.
Römisch K, Collie N, Soto N, Logue J, Lindsay M, Scheper W, Cheng CHC (2003). Protein translocation across the endoplasmic reticulum membrane in cold-adapted organisms. J Cell Sci 116, 2875-2283.
Wang T., Waters CT, Rothman AMK, Jakins TJ, Römisch K, Trump D (2002). Intracellular retention of mutant retinoschisin is the pathological mechanism underlying X-linked retinoschisis. Hum Mol Genet 11, 3097-105.
Harty C, Strahl S, Römisch K (2001). O-mannosylation protects mutant alpha-factor precursor from ER-associated degradation. Mol Biol Cell 12, 1093-1101.
Gillece P, Pilon M, Römisch K (2000). The protein translocation channel mediates glycopeptide export across the endoplasmic reticulum membrane. Proc Natl Acad Sci USA 97, 4609-4614.
Simpson JC, Roberts LM, Römisch K, Davey J, Wolf DH, Lord JM (1999). RicinA chain utilises the endoplasmic reticulum-associated degradation pathway to enter the cytosol. FEBS Lett 459, 80-84.
Gillece P, Luz JM, Lennarz WJ, de la Cruz FJ, Römisch K (1999). Export of a cysteine-free misfolded secretory protein from the ER for degradation requires interaction with protein disulfide isomerase. J Cell Biol 147, 1443-1456.
Pilon M, Römisch K, Quach D, Schekman R (1998). Cold-sensitive mutations in SEC61 cause defects in initiation of protein translocation into the yeast endoplasmic reticulum. Mol Biol Cell 9, 3455-3473.
Pilon M, Schekman R, Römisch K (1997). Sec61p mediates export of a misfolded secretory protein from the endoplasmic reticulum to the cytosol for degradation. EMBO J 16, 4540-4548.
Römisch K & Ali BRS (1997). Similar processes mediate glycopeptide export from the endoplasmic reticulum in mammalian cells and Saccharomyces cerevisiae. Proc Natl Acad Sci USA 94, 6730-6734.
Römisch K & Schekman R (1992). Distinct processes mediate glycoprotein and glycopeptide export from the endoplasmic reticulum in S. cerevisiae. Proc Natl Acad Sci USA 89, 7227-7231.
Killisch I., Steinlein P, Römisch K, Hollinshead R, Beug H, Griffiths G (1992). Characterization of early and late endocytic compartments of the transferrin cycle. Transferrin receptor antibody blocks erythroid differentiation by trapping the receptor in the early endosome. J Cell Sci 103, 211-232.
Lütcke H, High S, Römisch K, Ashford AJ, Dobberstein B (1992). The methionine-rich domain of the 54 kDa protein of signal recognition particle is sufficient for the interaction with signal sequences. EMBO J 11, 1543-1551.
Römisch K, Webb J, Lingelbach K., Gausepohl H, Dobberstein B (1990). The 54 kD protein of signal recognition particle contains a methionine-rich RNA binding domain. J Cell Biol 111, 1793-1802.
Ribes V, Römisch K, Giner A, Dobberstein B, Tollervey D (1990). E. coli 4.5S RNA is part of a ribonucleoprotein particle that has properties related to signal recognition particle. Cell 63, 591-600.
Prehn S, Herz J, Hartmann E, Kurzchalia TV, Frank R, Römisch K, Dobberstein B, Rapoport TA (1990). Structure and biosynthesis of the signal sequence receptor. Eur J Biochem 188, 439-445.
Reidl J, Römisch K, Ehrmann M, Boos W (1989). MalI, a novel protein involved in the regulation of the maltose system of Escherichia coli is highly homologous to the repressor proteins GalR, CytR, and LacI. J Bacteriol 171, 4888-4899.
McDowall A, Gruenberg J, Römisch K, Griffiths G (1989). The structure of organelles of the endocytic pathway in hydrated cryosections of cultured cells. Eur J Cell Biol 49, 281-294.
Römisch K, Webb J, Herz J, Prehn S, Frank R, Vingron M, Dobberstein B (1989). Homology of 54K protein of signal recognition particle, docking protein, and two E. coli proteins with putative GTP-binding domains. Nature 340, 478-482.
Alvira S, Corey RA, Collinson I, Römisch K (2020) Membrane protein biogenesis by the EMC. EMBO J 40(2):e107407.
Allen WJ, Collinson I, Römisch K (2019) Post-translational protein translocation by the Sec complex. Trends Biochem Sci April 5. doi: 10. 1016/j.tibs.2019.03.003.
Römisch K (2017). A case for Sec61 channel involvement in ER-associated degradation. Trends Biochem Sci 42:171-79.
Kalies KU & Römisch K (2015). Inhibitors of protein translocation across the ER membrane. Traffic 16, 1027-38.
Römisch K (2012). Diversion at the ER: how Plasmodium falciparum exports proteins into host erythrocytes.[v1: ref status:2, f1000res] F1000Research 1.
Römisch K (2006). The role of Ubx2p in ER-associated degradation. Trends Biochem Sci 31, 24-25.
Römisch K (2005). ER-associated degradation. Annu Rev Cell & Dev Biol 21, 435-456.
Römisch K (2005). Targeting signals in proteins involved in malaria. Traffic 6, 706-9.
Peck LS, Clark MS, Clarke A, Cockell CS, Convey P, Detrich III HW, Fraser KPP, Johnston IA, Methe BA, Murray AE, Römisch K, Rogers AD (2004). Genomics: Applications to Antarctic ecosystems. Polar Biology 28, 351-365.
Clark MS, Clarke A, Cockell CS, Convey P, Detrich III HW, Fraser KPP, Johnston IA, Methe BA, Murray AE, Peck LS, Römisch K, Rogers AD (2004). Antarctic Genomics. Comp Func Genomics 5, 230-238.
Römisch K (2004). A cure for traffic jams: small molecule chaperones in the endoplasmic reticulum. Traffic 5, 815-820.
Römisch K & Matheson T (2003). Cell biology in the Antarctic: studying life in the freezer. Nature Cell Biol 5, 3-6.
Römisch K (2003). Protein transport across the membrane of the endoplasmic reticulum. In Cell Biology: A Practical Approach, Davey J & Lord M (eds.), OUP, 151-163.
Römisch K (2002). Surviving extreme cold. BIF Futura 17, 216-221.
Römisch K (2002). Viral subversion of protein trafficking pathways. TiBS 27, 335; TCB 12, 360.
Römisch K (2002). Some like it hot. TiBS 27, 174.
Römisch K (2002). Garbage separation in the endoplasmic reticulum. TiBS 27, 7.
Römisch K (2001). Antibacterial defense relies on ER chaperone gp96. TiBS 26, 644.
Römisch K (2001). How antigenic peptides are made to fit their groove. TiBS 26,
531; Trends Biotechnol. 19, 378.
Römisch K (2001). Bulps switch the points in the Golgi complex. TiBS 26, 353.
Römisch K (2001). Microtubules: fast tracks to secretion? TiBS 26, 281.
Römisch K (2001). Contact: Interactions between ribosomes and protein translocation channels in the ER membrane. TiBS 26, 13.
Römisch K (2001). Contact: Interactions between ribosomes and protein translocation channels in the ER membrane. TiBS 26, 13.
Römisch K (2000). Ways to rein in wayward proteins: a novel mechanism for ER retention. TiBS 25, 541.
Römisch K (2000). Sugars pave the road to destruction. TiBS 25, 428.
Römisch K (2001). Molecular trafficking: are some organelles more important than others? J Cell Sci 114, 2359.
Lehner P, Hewitt EW, Römisch K (2000). MHC class I mediated antigen presentation: Proteins and peptides scramble for the exit. Curr Biol 10, R839-842.
Römisch K (1999) Surfing the Sec61 channel: Bidirectional protein translocation across the ER membrane. J. Cell Sci 112, 4185-4191.
Römisch K & Corsi A (1996). Protein translocation into the endoplasmic reticulum. In Protein Targeting, Hurtley S (ed), Oxford University Press, 101-122.
Römisch K (1994). Peptide traffic across the endoplasmic reticulum membrane - TAPs and other conduits. Trends Cell Biol 4, 311-314.
Römisch K, Ribes V, High S, Lütcke H, Tollervey D, Dobberstein B (1990). Structure and function of signal recognition particle (SRP). Mol Biol Reports 14, 71-72.
Over 300 short evaluations of published papers for Faculty of 1000 (since 2001).
