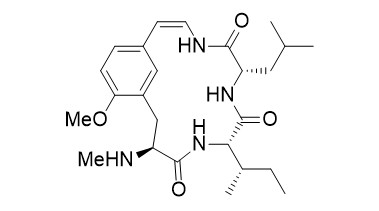
Abyssenine A
The abyssenines were isolated together with the mucronines from the bark of Zizyphus abyssinica. Some of these compounds were found to have significant antifungal and antibacterial activities. Structurally, they belong to a large family of cyclopeptide alkaloids with over 200 members.
- R. Tschesche, S. T. David, R. Zerbes, M. Radloff, E. U. Kausmann, G. Eckhardt, Liebigs Ann.Chem. 1974, 1915–1928.
- B. K. Cassels, G. Eckhardt, E.-U. Kaussmann, R.Tschesche, Tetrahedron 1974, 30, 2461–2466.
N-Methylated amino acids and peptides with an 8-aminoquinoline directing group can be subjected to stereoselective Pd-catalyzed β-functionalizations. The directing group can easily be removed, providing the free carboxylic acid, which can be used directly in peptide couplings. This protocol was used successfully as a key step for the introduction of the unusual side chain in the synthesis of the cyclopeptide alkaloids abyssenine A.
- T. Kinsinger, U. Kazmaier, "C-H-Functionalization of N-Methylated Amino Acids and Peptides as Tool in Natural Product Synthesis – Synthesis of Abyssenine A and Mucronine E", Org. Lett. 2018, 20, 7726–7730.