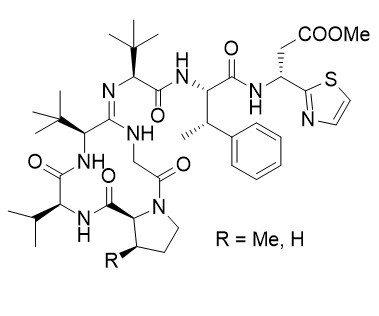
Bottromycin
Bottromycins were isolated from the fermentation broth of Streptomyces bottropensis and Streptomyces No. 3668-L2. This antibiotic inhibits the growth of a wide range of microorganisms by interfering with their protein biosynthesis. The unusual structure was determined correctly very recently.
- J. M. Waisvisz, M. G. van der Hoeven, J. van Peppen, W. C. M. Zwennis, J. Am. Chem. Soc. 1957, 79, 4520–4521.
- J. M. Waisvisz, M. G. van der Hoeven, J. F. Hölscher, B. Te Nijenhuis, J. Am. Chem. Soc. 1957, 79, 4522–4524.
- J. M.Waisvisz, J. M.; M. G. van der Hoeven, B. Te Nijenhuis, J. Am. Chem. Soc. 1957, 79, 4524–4527.
- T. Yamada et al., J. Org. Chem. 2018, 83, 13, 7135–7149.
Thio-Ugi reactions were found to be an excellent synthetic tool for the synthesis of sterically highly hindered endothiopeptides. S-Methylation and subsequent amidine formation can be carried out in an inter- as well as in an intramolecular fashion. The intramolecular approach allows the synthesis of the bottromycin ring system in a straightforward manner.
- S. Ackermann, H.-G. Lerchen, D. Häbich, A. Ullrich, U. Kazmaier, “Synthetic studies towards Bottromycin”, Beilstein J. Org. Chem. 2012, 8, 1652–1656.
- U. Kazmaier, "The Long, Long Way to Bottromycin", Isr. J. Chem. 2021, 61, 302–321. DOI: 10.1002/ijch.202000068.
- L. Franz, U. Kazmaier, A. W. Truman, J. Koehnke, "Bottromycins - Biosynthesis, Synthesis and Activity", Nat. Prod. Rep. 2021, 38, 1659–1683. DOI: 10.1039/d0np00097c.