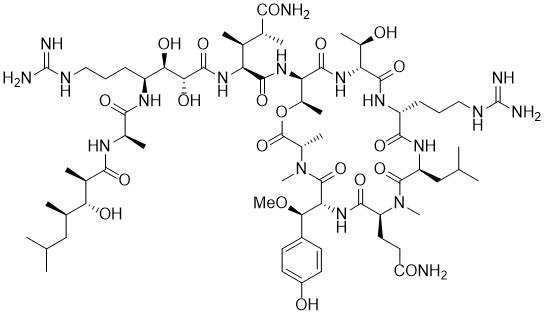
Callipeltin A
In 1996, Zampella and colleagues isolated the decapeptide Callipeltin A from Callipelta sp., a marine sponge found near the east coast of New Caledonia in the Pacific Ocean. Callipeltin A consists of a cyclic heptapeptide core and a highly functionalized sidechain. It contains several non-proteinogenic amino acids, as well as a rare terminal β-hydroxy acid. Callipeltin A displays interesting antiviral, antifungal, and cytotoxic activity.
- A. Zampella, M. V. D’Auria, L. Gomez Paloma, A. Casapullo, L. Minale, C. Debitus, Y. Henin, J. Am. Chem. Soc. 1996, 118, 6202–6209.
Matteson homologations of chiral boronic esters proved to be an excellent tool for the synthesis of highly functionalized amino acid residues. This method provides straightforward stereoselective access to the sidechain of callipeltin A as well as the allo-threonine of the peptide ring. Macrolactonisation of a heptapeptide precursor with PyAOP proved to be an excellent method for preparation of the cyclic depsipeptide ring of callipeltin A.
- A. Horn, U. Kazmaier, “Synthesis of the Cyclic Heptapeptide Core of Callipeltin A”, Org. Chem. Front. 2022, 9, 5213−5218. DOI: 10.1039/D2QO01120D.
- A. Horn, U. Kazmaier, “Stereoselective Synthesis of the Sidechain of Callipeltin A”, Org. Lett. 2022, 24, 7072–7076. DOI: 10.1021/acs.orglett.2c02586.