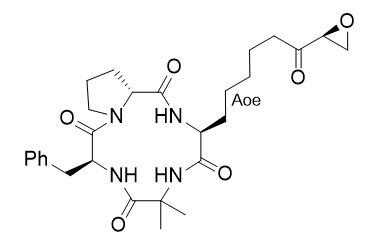
Chlamydocin
Chlamydocin belongs to an interesting family of cyclotetrapeptides containing Aoe [(2S,9S)-2-amino-8-oxo-9,10-epoxydecanoic acid], an unusual lipophilic amino acid with an epoxyketone functionality. Its molecular target is the histone deacetylase (HDAc), a nuclear isozyme that regulates gene transcription via a dynamic process of acetylation and deacetylation of lysine residues of histones. Blockade of the deacylating process causes hyperacetylation of histones and unregulated gene activity, that results in untimely cell death.
- A Closse, R. Huguenin, Helv. Chim. Acta 1974, 57, 533-545.
Chelate-Claisen rearrangement of a chiral allylic ester allows the synthesis of the unusual epoxyketo amino acid Aoe of Chlamydocin.
- C. Quirin, U. Kazmaier, “Synthesis of Chlamydocin via Chelate-Claisen Rearrangement”, Eur. J. Org. Chem. 2009, 371–377.