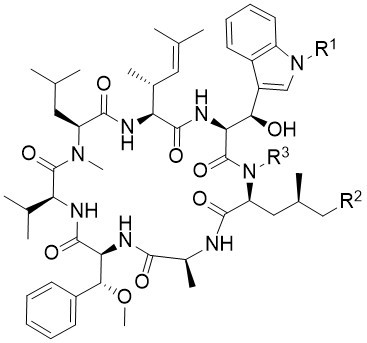
Cyclomarins
In 1999, Clardy et al. reported the isolation and structure elucidation of a family of new cyclopeptides from a marine streptomycete CNB-982, called cyclomarins. The major metabolite cyclomarin A showed an interesting activity toward mycobacterium tuberculosis. Detailed studies indicated, that ClpC1, a subunit of a caseinolytic protease, is the actual target of CycA. Cyclomarin also shows antimalaria activity by inhibiting the PfAp3Aase of the protozoan parasite.
- M. K. Renner, Y. C. Shen, X. C. Cheng, P. R. Jensen, W. Frankmoelle, C. A. Kauffman, W. Fenical, E. Lobkovsky, J. Clardy, J. Am. Chem. Soc. 1999, 121, 11273–11276.
- E. K. Schmitt et al., Angew. Chem. 2011, 123, 6011–6013, Angew. Chem. Int. Ed. 2011, 50, 5889–5891.
- N. Buerstner et al., ChemBioChem 2015, 16, 2433–2436.
Cyclomarins are cyclic heptapeptides containing four unusual amino acids. New synthetic protocols toward their synthesis have been developed, leading to the synthesis and biological evaluation of three natural occurring cyclomarins. Interestingly, cyclomarins address two completely different targets: Clp C1, a subunit of the caseinolytic protease of Mycobacterium tuberculosis (MTB), as well as PfAp3Ase of Plasmodium falciparum. Therefore, cyclomarins are interesting lead structures for the development of drugs against tuberculosis and malaria.
- P. Barbie, U. Kazmaier, "Synthesis of fully protected, reverse N-prenylated (2S,3R)-3-hydroxytryptophan, a unique building block of the cyclomarins", Org. Biomol. Chem. 2015, 13, 9267–9275.
- P. Barbie, U. Kazmaier, "Total Synthesis of Cyclomarin A, a Marine Cycloheptapeptide with anti-Tuberculosis and anti-Malaria Activity", Org. Lett. 2016, 18, 204−207.
- P. Barbie, U. Kazmaier, "Total synthesis of cyclomarins A, C and D, marine cyclopeptides with interesting anti tuberculosis and anti malaria activitites", Org. Biomol. Chem. 2016, 14, 6036−6054.
- P. Barbie, U. Kazmaier, "Total synthesis of desoxycyclomarin C and the cyclomarazines A and B", Org. Biomol. Chem. 2016, 14, 6055−6064.
- K. Weinhäupl, J. Lelievre, A. Goldberg, U. Kazmaier, P. Schanda, H. Fraga, "The natural antibiotic Cyclomarin blocks Arginine-Phosphate induced dynamics in ClpC1 N-terminal domain – A possible mechanism of drug action", J. Biol. Chem. 2018, 293, 8379−8393.
- A. Kiefer, U. Kazmaier, "Synthesis of modified β-methoxyphenylalanines via diazonium chemistry and their incorporation in desoxycyclomarin analogues", Org. Biomol. Chem. 2019, 17, 88–102.
- A. Kiefer, U. Kazmaier, "Syntheses of Cyclomarins – Interesting Marine Natural Products with Distinct Mode of Action towards Malaria and Tuberculosis", Synthesis 2019, 51, 107–121.
- A. Kiefer, C. D. Bader, J. Held, A. Esser, J. Rybniker, M. Empting, R. Müller, U. Kazmaier, "Synthesis of New Cyclomarin Derivatives and Their Biological Evaluation towards Mycobacterium Tuberculosis and Plasmodium Falsiparum", Chem. Eur. J. 2019, 25, 8894–8902.
- U. Kazmaier, L. Junk, "The Synthesis of Ilamycins/Rufomycins and Cyclomarins, Marine Cyclopeptides that Demonstrate anti-Malaria and anti-Tuberculosis Activity", Mar. Drugs 2021, 19, 446. DOI: 10.3390/md19080446.
- F. E. Morreale, S. Kleine, J. Leodolter, S. Junker, D. M. Hoi, S. Ovchinnikov, A. Okun, J. Kley, R. Kurzbauer, L. Junk, S. Guha, U. Kazmaier, G. Boehmelt, D. Kessler, K. Rumpel, M. Hartl, D. Haselbach, A. Meinhart, M. Kaiser, T. Clausen, “BacPROTACs mediate targeted protein degradation in bacteria“, Cell 2022, 185, 2338−2353. DOI: 10.1016/j.cell.2022.05.009.
- D. M. Hoi, S. Junker, L. Junk, K. Fischel, D. Podlesainski, K. Schwechel, F. Kasani, L. van Geelen, J. Leodolter, P. Hawkins, F. E. Morreale, S. Kleine, S. Guha, K. Rumpel, V. M. Schmiedel, H. Weinstabl, A. Meinhart, R. Kalscheuer, R. J. Payne, M. Kaiser, M. Hartl, G. Boehmelt, U. Kazmaier, T. Clausen, "Development of a dual Clp-targeting BacPROTAC that impairs mycobacterial proteostasis and survival", Cell 2023, 186, 2176–2192. DOI: 10.1016/j.cell.2023.04.009.
- L. Junk, V. M. Schmiedel, S. Guha, K. Fischel, P. Greb, K. Schwechel, V. Krisilia, L. van Geelen, K. Rumpel, P. Kaur, R. V. Krishnamurthy, S. Narayanan, C. Kofink, A. Mantoulidis, P. Biber, G. Gmaschitz, U. Kazmaier, A. Meinhart, J. Leodolter, D. Hoi, S. Junker, F. E. Morreale, T. Clausen, R. Kalscheuer, H. Weinstabl, G. Boehmelt, "Homo-BacPROTAC-induced degradation of ClpC1 as a strategy against drug-resistant mycobacteria", Nature Commun. 2024, 15, 2005. DOI: 0.1038/s41467-024-46218-7.