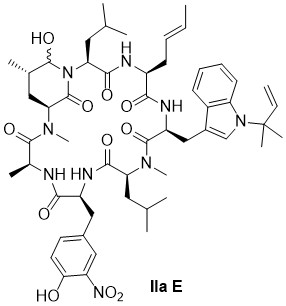
Ilamycin
Ilamycins/rufomycins are marine cycloheptapeptides containing unusual amino acids. Produced by Streptomyces sp. and isolated in 1962, these compounds show potent activity against a range of mycobacteria, including multidrug-resistant strains of Mycobacterium tuberculosis. The cyclic peptides target the AAA+ protein ClpC1 that, together with the peptidases ClpP1/ClpP2, forms an essential ATP-driven protease.
- Y. Nakayama, T. Takita, H. Ozawa, H. Umezawa, K. Tahara, J. Antibiot. 1962, 15, 49–50.
- T. Takita, K. Ohi, K. Maeda, Y. Okami, H. Umezawa, J. Antibiot. 1962, 15, 46–48.
- M. Shibata, H. Yamamoto, E. Higashidani, K. Nakazawa, Agric. Biol. Chem. 1962, 26, 228–233.
Derivatives of the ilamycins with a simplified tryptophane unit are synthesized in a straightforward manner. A ilamycin derivative with a cyclic hemiaminal structure is active in the nM-range against several mycobacterial strains and shows no significant cytotoxicity. Detailed investigations of the mode of action of indicate that itderegulates ClpC1 activity and strongly enhances ClpC1-WT ATPase activity.
U. Kazmaier, L. Junk, “The Synthesis of Ilamycins/Rufomycins and Cyclomarins, Marine Cyclopeptides that Demonstrate anti-Malaria and anti-Tuberculosis Activity”, Mar. Drugs 2021, 19, 446. DOI: 10.3390/md19080446.
J. Greve, A. Mogk, U. Kazmaier, “Total Synthesis and Biological Evaluation of Modified Ilamycin Derivatives”, Mar. Drugs 2022, 20, 632. DOI: 10.3390/md20100632.