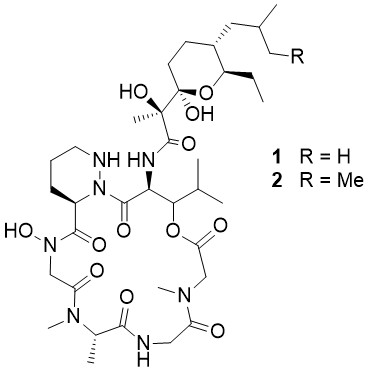
Meliponamycin A
Pupo et al. described the isolation of the meliponamycins, new cyclohexadepsipeptides isolated from Streptomyces sp. ICBG1318, which is associated with M. scutellaris nurse bees. Both compounds, meliponamycin A (1) and meliponamycin B (2), show strong activity against human pathogens, such as Staphylococcus aureus and Leishmania infantum. The most interesting structural feature is a cyclized polyketide fragment containing a tetrahydropyranyl (THP) ring system connected to the piperazic acid β-hydroxyleucine dipeptide fragment of the depsipeptide ring.
- C. Menegatti, V. B. Lourenzon, D. Rodríguez-Hernández, W. G. da Paixão Melo, L. L. G. Ferreira, A. D. Andricopulo, F. S. do Nascimento, M. T. Pupo, J. Nat. Prod. 2020, 83, 610–616.
The Matteson homologation was found to be a versatile tool for the construction of the linear polyketide side chain of Meliponamycin, and related compounds, in only four steps. The ester dienolate version of this reaction allowed the introduction of the unsaturated ester moiety in a highly stereoselective fashion. Boronate oxidation/ desoxygenation and Sharpless dihydroxylation are additional key steps in the stereoselective construction of this highly functionalized tetrahydropyran ring system, which is characteristic of this substance class.
- O. Andler, U. Kazmaier, "Stereoselective Synthesis of a Protected Side Chain of Meliponamycin A", Org. Lett. 2022, 24, 2541−2545. DOI: 10.1021/acs.orglett.2c00701.
- O. Andler, U. Kazmaier, "Matteson Homologation-Based Total Synthesis of Meliponamycin A", Org. Lett. 2024, 26, 148–152. DOI: 10.1021/acs.orglett.3c03766 .