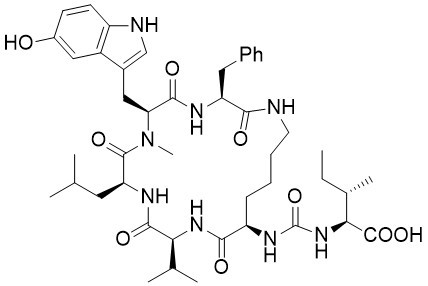
Mozamide A
The cyclic peptides mozamide A and B were isolated in 1997 by Faulkner et al. from a sponge of genus Theonella, which had been collected in Mozambique. The mozamides belong to the large family of anabaenopeptin-type peptides, which show a wide range of biological properties. The structures of the mozamides were elucidated via NMR and MS experiments and the configurations of the constituting amino acids were determined by degradation of the peptides followed by derivatization and chiral GC analysis.
- E. W. Schmidt, M. K. Harper, D. Faulkner, J. Nat. Prod. 1997, 60, 779–782.
Mozamide A was synthesized for the first time via a convergent and flexible route. The installation of the substituted tryptophan moieties was accomplished at the very end of the synthesis and thus allows easy modifications at this position. Comparison of the NMR data of the synthesized cyclopeptide with the natural product clearly indicates, that the originally proposed structure of mozamide A cannot be correct. The synthesis of two other diastereomers allowed the correction of the configuration of three amino acid building blocks. Mozamide A contains L-Val, D-Lys and L-Ile (instead of D-Val, L-Lys and L-allo-Ile) and is a hydroxylated brunsvicamide.
- L. Junk, U. Kazmaier, "Total Synthesis and Configurational Revision of Mozamide A – a Hydroxy-Brunsvicamide", J. Org. Chem. 2019, 84, 2489–2500.