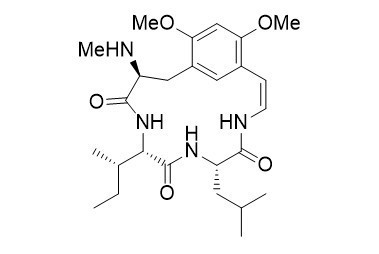
Mucronine E
Cyclopeptide alkaloids are a group of closely related polyamide bases of plant origin. The mucronines were isolated together with the abyssenines from the bark of Zizyphus abyssinica and were found to have significant antifungal and antibacterial activities. Structurally speaking, they possess a 13-, 14-, or 15-membered cycle containing a peptide unit connected to an aromatic ring by an enamide linkage.
- H.-W. Fehlhaber, J. Uhlendorf, S. T. David, R. Tschesche, Liebigs Ann. Chem. 1972, 759, 195–297.
- N. H. Tan, J. Zhou, Chem. Rev. 2006, 106, 840–895.
N-methylated amino acids and peptides with an 8-aminoquinoline (AQ) directing group can be subjected to stereoselective Pd-catalyzed β-functionalizations. The AQ protecting group can easily be removed, providing the free carboxylic acid, which can be used directly in peptide couplings. This protocol was used successfully as a key step in the synthesis of the cyclopeptide alkaloids mucronine E.
- T. Kinsinger, U. Kazmaier, "C-H-Functionalization of N-Methylated Amino Acids and Peptides as Tool in Natural Product Synthesis – Synthesis of Abyssenine A and Mucronine E", Org. Lett. 2018, 20, 7726–7730.