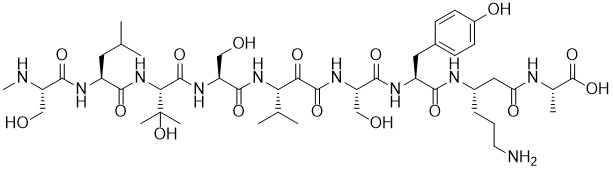
Myxoprincomide
Modern genome mining techniques, combining targeted mutagenesis, liquid chromatography coupled with HRMS, and statistical data analysis identified myxoprincomide as a new secondary metabolite from Myxococcus xanthus DK 1622. The linear nonapeptide contains some unusual building blocks such as β-hydroxylated valine, β-lysine and an α-keto-β-amino acid derived from valine. The isolated amounts allow for the de novo structure elucidation of a new natural product, but only few investigations of the biological activities.
- N. S. Cortina, D. Krug, A. Plaza, E. Revermann and R. Müller, Angew. Chem. 2012, 124, 836–841; Angew. Chem. Int. Ed. 2012, 51, 811–816.
Myxoprincomide is synthesised for the first time. The central, unusual α-ketoamide is generated at the end of the synthesis to avoid side reactions during the synthesis of this rather reactive subunit. Nevertheless, the synthetic natural product is obtained as an isomeric mixture. Detailed analytical investigations show that the identical isomeric mixture is found in the isolated natural product.
- M. Kohr, C. Walt, J. Dastbaz, R. Müller, U. Kazmaier, "Total Synthesis of Myxoprincomide, a secondary metabolite from Myxococcus xanthus", Org. Biomol. Chem. 2022, 20, 9609–9612. DOI: 10.1039/D2OB02021A.