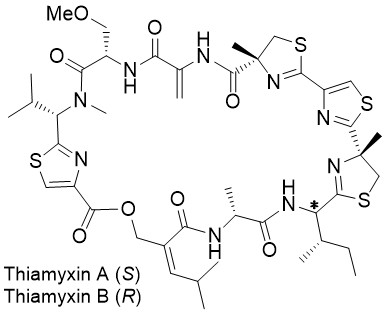
Thiamyxins
Modern genome mining techniques, combining targeted mutagenesis, liquid chromatography coupled with HRMS, and statistical data analysis identified myxoprincomide as a new secondary metabolite from Myxococcus xanthus DK 1622. The linear nonapeptide contains some unusual building blocks such as β-hydroxylated valine, β-lysine and an α-keto-β-amino acid derived from valine. The isolated amounts allow for the de novo structure elucidation of a new natural product, but only few investigations of the biological activities.
- P. A. Haack, K. Harmrolfs, C. D. Bader, R. Garcia, A. P. Gunesch, S. Haid, A. Popoff, A. Voltz, H. Kim, R. Bartenschlager, T. Pietschmann, R. Müller, Angew. Chemie Int. Ed. 2022, 61, e202212946.
The first total synthesis of the thiamyxins A-C and the new thiamyxin E features a parallel closing of two methyl thiazoline units, with low epimerization of the very labile adjacent stereocenter. It also includes the three-step synthesis of an uncommon hydroxy acid and the oxidation-free elimination of a phenylselenide to form a dehydroalanine moiety. The exploitation of the acid labile stereocenter at the isoleucine moiety and the reopening of the macrolactones gave access to the four thiamyxins with good yields and diastereomeric purities from a single precursor.
- K. Bauer, U. Kazmaier, "First Total Synthesis of Thiamyxins A-C and Thiamyxin E, a Potent Class of RNA-Virus Inhibiting (Cyclo)depsipeptides", Angew. Chem. 2023, 135, e202305445. DOI: 10.1002/anie.202305445.