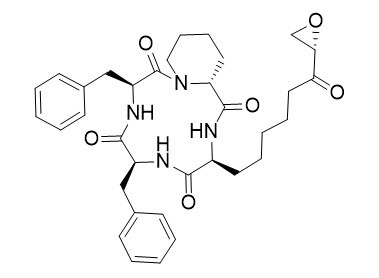
Trapoxin A
Posttranslational reversible histone acetylation at the ε-amino groups of conserved lysins plays a major role in the regulation of gene expression. Luckily, a wide range of macrocyclic peptides have been identified as naturally occurring HDAC inhibitors. For example, trapoxin, a fungal metabolite from Helicoma ambiens RF-1023, was found to cause accumulation of highly acetylated histones in various mammalian cell lines by irreversible inhibition of the deacetylating enzymes.
- H. Itazaki et al., J. Antibiot. 1990, 43, 1524–1532.
Peptide modification reactions, e.g. via Palladium- catalyzed allylic alkylations, are useful tools for the synthesis of peptides containing interesting non-proteinogenic amino acids, which are often essential for the biological activity of natural products and drugs. The potential of this protocol is demonstrated in the first total synthesis of HDAC inhibitor trapoxin A.
- P. Servatius, U. Kazmaier, "Total Synthesis of Trapoxin A, a fungal HDAC-inhibitor from Helicoma ambiens", J. Org. Chem. 2018, 83, 11341−11349.