Synthesis of Amino Acids and Peptides
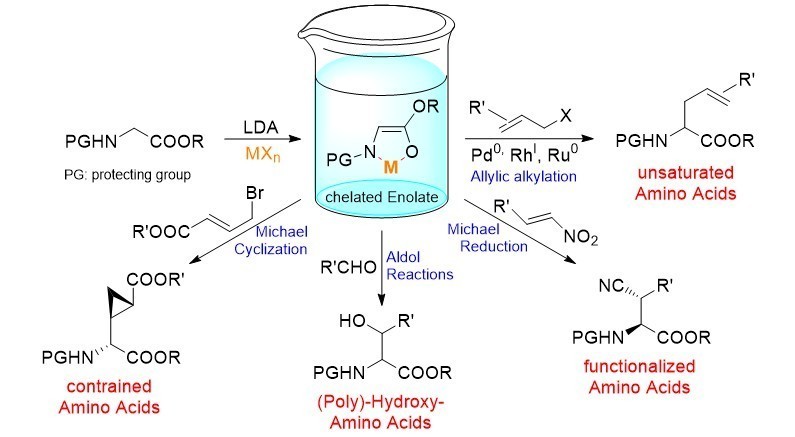
The group is involved in the synthesis of highly functionalized and complex amino acids. We are mainly focussing on reactions of chelated amino acid ester enolates, proceeding in a highly stereoselective fashion because of a fixed enolate geometry. Besides classical enolate reactions, such as aldol and Michael additions, also reactions become possible which can’t be carried out with “normal”, non-chelated enolates, such as Claisen rearrangements and transition metal-catalyzed allylic alkylations. The introduction of metallated unsaturated side chains allows successive modifications using cross coupling chemistry.
These methods are not limited to amino acids, but can also be applied to peptides. This allows the introduction of unsaturated side chains into a given peptide. Via multifold allylations, followed by ring closing metatheses cyclic peptides can be obtained. This approach can also be applied in natural product syntheses.