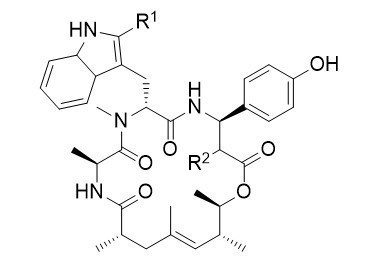
Chondramide Derivatives
In 1995 Höfle and Reichenbach reported the isolation of the chondramides, cyclodepsipeptides closely related to jasplakinolide and geodiamolide, mainly differing in the polyketide fragment. The chondramides where shown to bind to actin in an analogous manner like these peptides, what makes this substances also highly interesting from a pharmaceutical point of view.
- B. Kunze, R. Jansen, F. Sasse, G. Höfle, H. Reichenbach, J. Antibiot. 1995, 48, 1262–1266.
- R. Jansen, B. Kunze, H. Reichenbach, G. Höfle, Liebigs Ann. 1996, 285–290.
Removing the methyl groups and the stereogenic centers from the ω-hydroxyacid of the chondramides results in a significant drop in the cytotoxicity of these interesting depsipeptides. This effect can be almost compensated by introduction of a second chlorine atom on the β-tyrosine moiety of the natural products. These simplified chondramides are much easier accessible than the natural chondramides.
- D. Becker, U. Kazmaier, "Synthesis of Simplified Halogenated Chondramide Derivatives as new Actin-binding Agents", Eur. J. Org. Chem. 2015, 2591–2602.
- D. Becker, U. Kazmaier, "Synthesis and Biological Evaluation of Dichlorinated Chondramide Derivatives", Eur. J. Org. Chem. 2015, 4198–4213.