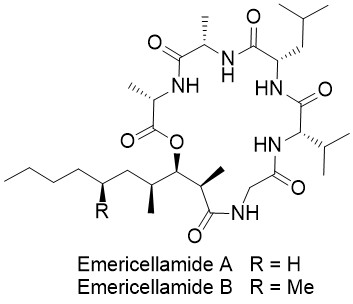
Emericellamide A
Emericellamide A and B are two cyclic lipopeptides that were isolated from the marine-derived fungus Emericella sp. by Fenical et al. in 2007. These natural products are produced in such tiny amounts that they were not detectable by standard LC-MS techniques. Biological studies indicated that the emericellamides show activity against methicillin-resistant Staphylococcus aureus (MRSA) strains as well as moderate cytotoxicity against HCT-116 cells.
- D.-C. Oh, C. A. Kauffman, P. R. Jensen, W. Fenical, J. Nat. Prod. 2007, 70, 515–520.
The Matteson homologation is the perfect approach for the synthesis of polyketide–peptide natural products such as the emericellamides. In only 4 steps the polyketide fragment with three stereogenic centers can be obtained as a single stereoisomer. The peptide fragment is easily available via solid-phase peptide synthesis.
- R. Priester, U. Kazmaier, "A Straightforward Synthesis of Emericellamide A using Matteson’s Homologation Approach", Synlett 2023, 34, 2159-2164. DOI: 10.1055/a-2077-2113