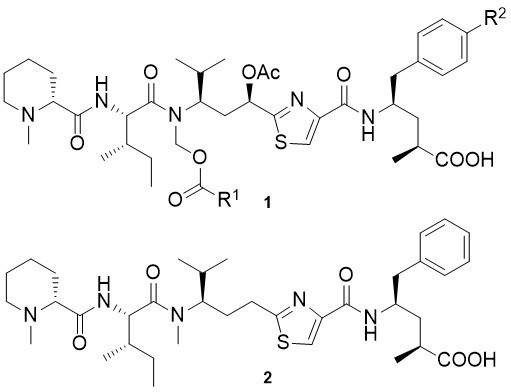
Tubulysin Derivatives
The tubulysins (1) are a family of nine secondary metabolites produced by several strains of myxobacteria. They are among a handful of natural products which interact with the eukaryotic cytoskeleton, inhibiting the polymerization of tubulin at very low concentrations (<50 pg mL-1). Notably, their ability to suppress the growth of cancerous cells exceeds that of other tubulin modifiers, including the epothilones, vinblastine and taxol.
- F. Sasse, H. Steinmetz, J. Heil, G. Höfle, H. Reichenbach, J. Antibiot. 2000, 53, 879–885.
- H. Steinmetz, N. Glaser, E. Herdtweck, F. Sasse, H. Reichenbach, G. Höfle, Angew. Chem. 2004, 116, 4996–5000; Angew. Chem. Int. Ed. 2004, 43, 4888–4892.
Pretubulysin (2), a biosynthetic precursor of the tubulysins, shows potent biological activity in the subnanomolar range towards various tumor cell lines. Its activity is only slightly reduced relative to the structurally more complex tubulysins. Pretubulysin induces apoptosis and inhibits cancer cell migration and tubulin assembly in vitro. With a straightforward synthesis in hand, pretubulysin is an ideal lead structure for the development of tubulysin-based anti-cancer drugs.
- A. Ullrich, Y. Chai, D. Pistorius, Y. A. Elnakady, J. E. Herrmann, K. J. Weissman, U. Kazmaier, R. Müller, "Pretubulysin, a potent and chemically-accessible tubulysin precursor from Angiococcus disciformis", Angew. Chem. 2009, 121, 4486–4489; Angew. Chem. Int. Ed. 2009, 47, 4422–4425.
- A. Ullrich, J. Herrmann, R. Müller, U. Kazmaier, "Synthesis and biological evaluation of pretubulysin and derivatives", Eur. J. Org. Chem. 2009, 6367–6378.
- Y. Chai, D. Pistorius, A. Ullrich, K. J. Weissman, U. Kazmaier, R. Müller, "Discovery of 23 Novel Natural Tubulysins from Angiococcus disciformis An d48 and Cystobacter SBCb004", Chem. Biol. 2010, 17, 296–309.
- J. L. Burkhart, R. Müller, U. Kazmaier, "Syntheses and Evaluation of Simplified Pretubulysin Analogues", Eur. J. Org. Chem. 2011, 3050–3059.
- J. L. Burkhart, U. Kazmaier, "A straightforward click-approach towards pretubulysin-analogues", RSC Advances 2012, 2, 3785–3790.
- J. Herrmann, R. M. Wiedmann, Y. A. Elnakady, A. Ullrich, M. Rohde, U. Kazmaier, A. M. Vollmar, R. Müller, "Pretubulysins: from a hypothetical biosynthetic intermediate to potential lead in tumor therapie", PLoS One 2012, 7, e37416, 1–12.
- J. Eirich, J. L. Burkhart, A. Ullrich, A. Vollmar, S. Zahler, U. Kazmaier, S. A. Sieber, "Pretubulysin derived probes as novel tools for monitoring the microtubule network via activity-based protein profiling and fluorescence microscopy", Mol. BioSyst. 2012, 8, 2067–2075.
- S. Rath, J. Liebl, R. Fürst, A. Ullrich, J. L. Burkhart, U. Kazmaier, J. Herrmann, R. Müller, M. Günther, L. Schreiner, E. Wagner, A. M. Vollmar, S. Zahler, "Anti-angiogenic effects of the tubulysin precursor pretubulysin and of simplified pretubulysin derivatives", Brit. J. Pharmacol. 2012,167, 1048–1061.
- U. Kazmaier, A. Ullrich, J. Hoffmann, "Synthetic Approaches towards Tubulysins and their Derivatives", Open Nat. Prod. J. 2013, 6, 12−30.
- V. K. Kretzschmann, D. Gellrich, A. Ullrich, S. Zahler, A. M. Vollmar, U. Kazmaier, R. Fürst, "The novel tubulin antagonist pretubulysin exhibits profound tumor vessel disrupting properties in vitro and in vivo", Arterioscl. Thromb. Vasc. Biol. 2014, 34, 294–303.
- S. Braig, R. M. Wiedmann, J. Liebl, M. Singer, R. Kubisch, L. Schreiner, B. A. Abhari, E. Wagner, U. Kazmaier, S. Fulda, A. M. Vollmar, "Pretubulysin – a new option for the treatment of metastatic cancer", Cell Death Disease 2014, 5, e1001.
- R. Kubisch, M. von Gamm, S. Braig, A. Ullrich, J. L Burkhart, L. Colling, J. Hermann, O. Scherer, R. Müller, O. Werz, U. Kazmaier, A. M. Vollmar, "Simplified pretubulysin-derivatives and their biological effects on cancer cells", J. Nat. Prod. 2014, 77, 536–542.
- J. Hoffmann, U. Kazmaier, "A Straightforward Approach towards Cyclic Photoactivatable Tubulysin Derivatives", Angew. Chem. 2014, 126, 11538–11542; Angew. Chem. Int. Ed. 2014, 53, 11356–11360.
- J. Hoffmann, J. Gorges, L. Junk, U. Kazmaier, "Synthesis of Pretubulysin-Derivatives via the TubUgi-Approach", Org. Biomol. Chem. 2015, 13, 6010–6020.
- I. Trübenbach, J. Gorges, J. Kuhn, E. Baratti, U. Kazmaier, E. Wagner, U. Lächelt, "Sequence-Defined Oligoamide Drug Conjugates of Pretubulysin and Methotrexate for Folate Receptor Targeted Cancer Therapy", Macromol. Biosci. 2017, 17, 1600520.
- R. Schwenk, T. Stehning, I. Bischoff, A. Ullrich, U. Kazmaier, R. Fürst, "The anti-metastatic action of the microtubule-targeting agent pretubulysin is associated with the trapping of tumor cells to the endothelium", Oncotarget 2017, 77622–77633.
- S. Kern, I. Trübenbach, M. Höhn, J. Gorges, U. Kazmaier, S. Zahler, A. M. Vollmar, E. Wagner, "Combined Antitumoral Effects of Pretubulysin and Methotrexate", Pharmacol. Res. Persp. 2019, e00460.
- I. Truebenbach, S. Kern, D. Loy, J. Gorges, U. Kazmaier, E. Wagner, "Combination chemotherapy of L1210 tumors in mice with pretubulysin and methotrexate nanomicelles", Mol. Pharmaceutics 2019, 16, 2405−2417.
- I. Truebenbach, W. Zhang, Y. Wang, S. Kern, M. Höhn, S. Reinhard, J. Gorges, U. Kazmaier, E. Wagner, "Co-delivery of pretubulysin and siEG5 to EGFR 2 overexpressing carcinoma cells", Int. J. Pharm. 2019, DOI: 10.1016/j.ijpharm.2019.118570.