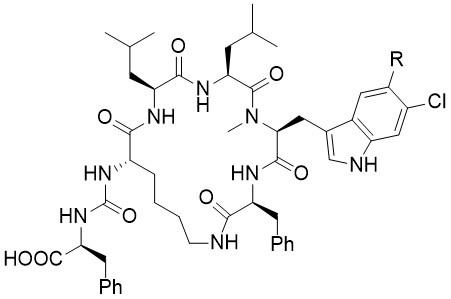
Keramamides
The keramamides were isolated in 1991 and 1996 respectively by Kobayashi et al. from a marine Theonella sponge. The keramamides belong to the larger class of anabaenopeptin-type peptides, which exhibit a range of interesting biological activities. Unfortunately, several structure elucidations of these natural products are not correct, proposing either wrong stereogenic centers or wrong amino acids.
- J. Kobayashi, M. Sato, M. Ishibashi, H. Shigemori, T. Nakamura, Y. Ohizumi, J. Chem. Soc., Perkin Trans. 1 1991, 2609–2611.
- H. Uemoto, Y. Yahiro, H. Shigemori, M. Tsuda, T. Takao, Y. Shimonishi, J. Kobayashi, Tetrahedron 1998, 54, 6719–6724.
The Keramamides A and L were synthesized for the first time via a convergent and flexible route. The installation of the substituted tryptophan moieties was accomplished at the very end of the synthesis on the cyclic peptides and thus enabled the synthesis of both natural products from one common precursor. The preparation of several epimers clearly indicates that the originally proposed relative configurations of both Keramamides A and L was not correct.
- L. Junk, U. Kazmaier, "Total Synthesis of Keramamides A and L from a Common Precursor by Late-Stage Indole Synthesis and Configurational Revision", Angew. Chem. 2018, 130, 11602−11606; Angew. Chem. Int. Ed. 2018, 57, 11432−11435.