Investigating Sex-Based Variations in Effector Cytotoxic T Lymphocytes Function in Cancer, Autoimmunity, and Inflammation
This research investigates the impact of sex-based variations on cytotoxic T lymphocytes (CTLs) in key areas of human health: cancer, autoimmune disorders, and inflammation. CTLs play a crucial role in adaptive immunity, exhibiting sex-specific immune responses that influence susceptibility to diseases such as cancer and autoimmune disorders. Females often display a robust and rapid immune response to infections, but this heightened reactivity also increases susceptibility to autoimmune diseases.
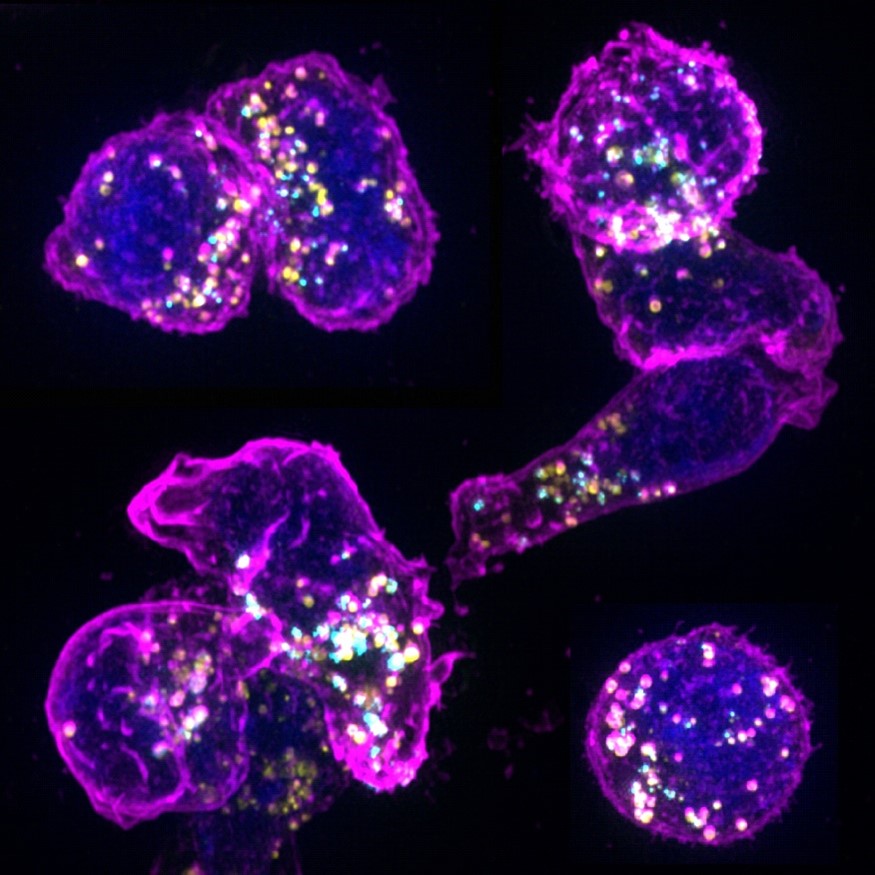
CTLs utilize cytotoxic granules (CGs), which contain lytic substances, to eliminate recognized viral-infected and cancer cells by inducing target cell death. Recently, we identified a novel type of CGs known as multicore cytotoxic granules (MCGs), characterized by unique morphology and composition. MCGs encapsulate lytic supramolecular attack particles (SMAPs), exosomes, and cytokines, highlighting their multifunctional potential.
Given the significant impact of sex on immune responses and disease susceptibility, our research investigates sex differences in CTLs' effector function concerning MCG production and utilization. We aim to understand how T cells strategically use MCGs as a multifunctional bioweapon in target cell killing, cellular communication, and functional adaptation across various pathological environments.

Project lead
Dr. Hsin-Fang Chang
Saarland University
Center for Integrative Physiology and Molecular Medicine (CIPMM)
Cellular neurophysiology
Important publications
- Lin CH, Scheller A, Liu Y, Krause E, Chang HF (2023). Study of Effector CD8+ T Cell Interactions with Cortical Neurons in Response to Inflammation in Mouse Brain Slices and Neuronal Cultures. Int J Mol Sci. 24(4):3166.
- Fang LP, Zhao N, Caudal LC, Chang HF, Zhao R, Lin CH, Hainz N, Meier C, Bettler B, Huang W, Scheller A, Kirchhoff F, Bai X (2022). Impaired bidirectional communication between interneurons and oligodendrocyte precursor cells affects social cognitive behavior. Nat. Commun. 13(1):1394.
- Chang HF*, Schirra C, Ninov M, Hahn U, Ravichandran K, Krause E, Becherer U, Bálint Š, Harkiolaki M, Urlaub H, Valitutti S, Baldari CT, Dustin ML, Jahn R, and Rettig J* (2022). Identification of distinct cytotoxic granules as the origin of supramolecular attack particles in T lymphocytes. Nat. Commun. 13(1):1029. *corresponding author.
- Chang HF*, Wirkner ML, Krause E, Rettig J* (2020). Investigation of Cytotoxic T Lymphocyte Function during Allorejection in the Anterior Chamber of the Eye. Int. J. Mol. Sci. 21. 4660. *corresponding author.
- Lodygin D, Hermann M, Schweingruber N, Flügel-Koch C, Watanabe T, Schlosser C, Merlini A, Körner H, Chang HF, Fischer HJ, Reichardt HM, Zagrebelsky M, Mollenhauer B, Frahm J, Stadelmann C, Kügler S, Fitzner D, Haberl M, Odoardi F, Flügel A (2019). β-Synuclein reactive T cells induce autoimmune CNS grey matter degeneration. Nature 566, 503-508.
- Chang HF, Mannebach S, Beck A, Ravichandran K, Krause E, Frohnweiler K, Fecher-Trost C, Schirra C, Pattu V, Flockerzi V, Rettig J (2018). Cytotoxic granule exocytosis depends on the Flower protein. J. Cell Biol. 217, 667-683.
- Chang HF, Bzeih H, Schirra C, Chitirala P, Halimani M, Cordat E, Krause E, Rettig J, Pattu V (2016). Endocytosis of cytotoxic granules is essential for multiple killing of target cells by T lymphocytes. J. Immunol. 197, 2473-2484
- Matti U, Pattu V, Halimani M, Schirra C, Krause E, Liu Y, Weins L, Chang HF, Guzman R, Olausson J, Freichel M, Schmitz F, Pasche M, Becherer U, Bruns D, Rettig J (2013). Synaptobrevin2 is the v-SNARE required for cytotoxic T lymphocyte lytic granule fusion. Nat. Commun. 4, 1439