Motivation, Research & Group Life
Synopsis
We are a synthetic group interested in molecular inorganic and coordination chemistry, energy conversion and organic materials. We focus on methodology and mechanisms including the isolation of reactive intermediates, their bond-activation chemistry (targeting eventually catalysis), and their spectroscopic properties in the context of optoelectronics. We use computational chemistry to guide our synthetic efforts. We are passionate about any type of inorganic and organic chemistry, because we believe that all elements are made equal. Typically, our research involves carbenes and molecules with small frontier orbital energy gaps such as radicals, diradicals and zwitterions. We aim at creative and innovative approaches for long-standing challenges.
We believe that good science is a team effort, and thus are involved in many national and international collaborations. Current and previous external partners comprise:
- Guy Bertrand (UC San Diego, USA)
- Daniel Mindiola (UPenn, USA)
- Rodolphe Jazzar (San Diego, USA)
- Jiaxiang Chu (CAS Beijing)
- David Martin (Université Grenoble Alpes, France)
- Ludovic Favereau (Université Rennes 1, France)
- Marc Mauduit (ENSC Rennes, France)
- Andrea Biffis (Universitá di Padova, Italy)
- Stephan Hohloch (Universität Innsbruck, Austria)
- Max Hansmann (TU Dortmund)
- Shigeyoshi Inoue (TU München)
- Alexander Pöthig (TU München)
- Karsten Meyer (FAU Erlangen)
- Alejandro Cadranel (FAU Erlangen)
- Romano Dorta (FAU Erlangen)
- Dirk Guldi (FAU Erlangen)
- Kallol Ray (HU Berlin)
- Sergio Jannuzzi (MPI CEC)
- Gunnar Werncke (Universität Marburg)
- Christian Hering-Junghans (LIKAT Rostock)
- …
- Fumehoods equipped with Schlenklines under Ar and N2
- Three 4-glove dryboxes
- Potentiometry, CV, coulometry
- UV-Vis/NIR, in-situ UV-Vis
- Luminescence (thanks to G. Kickelbick)
- Low-T UV-Vis (thanks to D. Scheschkewitz)
- Ball mills
- Benchtop-EPR (thanks to C. Kay)
- Elemental Analysis
- IR, Raman
- Solvent-Purification-System (SPS), Stills
- Separate Office Space
- State-of-the-art mass spectrometry facilities
- State-of-the-art NMR facilities including Cryo-Probe and Solid-State instrument
- State-of-the-art SC/P-XRD facilities

We have proposed and demonstrated how to tame diradicals and Moebius-cumulenes with carbenes for application in singlet-fission, alleged technology of future solar cells with unrivaled quantum efficience, and two-photon absorption, key for biomedical imaging and signal transmission. Similarly, we explored what renders carbene-radicals (i) persistent toward disproportionation and (ii) generally thermodynamically stable. Current work is dedicated to both areas as well as rendering organic radicals, commonly highly air-sensitive, sufficiently stable for practical applications. In a side project, we have discovered bright luminescent complexes of s-block (!) metals. Joint-work with the Bertrand group presented the first isolable secondary C-radical.
We have a long-standing interest in the mechanistic intricacies of late transition metal catalysis. Using a large variety of physical-organic and physical-inorganic techniques, we have shown how Pd(0) complexes may oxidatively add amines, alcohols, CC bonds, and water, previously though to be unfeasible. Our findings suggest how to achieve additive-free and thus "green" cross-coupling catalysis. We furthermore propose anti-Dewar-Chatt-Duncanson umpolung by palladium(0) for Lewis-base activation of olefins, arenes and organic carbonyls and demonstrated catalytic CF/CH bond metathesis.

We have a long-standing interest in multiple-bonding “beyond the oxo-wall” and corresponding unusual electronic structures, which allow for the swift activation of strong bonds in redox-catalysis. Thereby, we have proposed that charge-separation, viz. zwitterionic character in π-systems, is a general motif for bond activations. We thereby presented the first examples for palladium imides and singlet nitrenes. Recently, we have direct our attention toward anionic terminal imides of gold(I) and free nitrenes. Further contributions with the Meyer-group include iron- and cobalt terminal imido complexes as well as nitrenes, and even an iron(VII) (yes, VII!) nitrido-complex.

We presented a new approach toward synthesizing electron-precise and zerovalent nanoclusters stabilized by bridging phosphinidene ligands. Current work aims at high-spin congeners, their embedment into metal-organic frameworks, as well as corresponding nanowires (joint work with T. Kraus @INM).
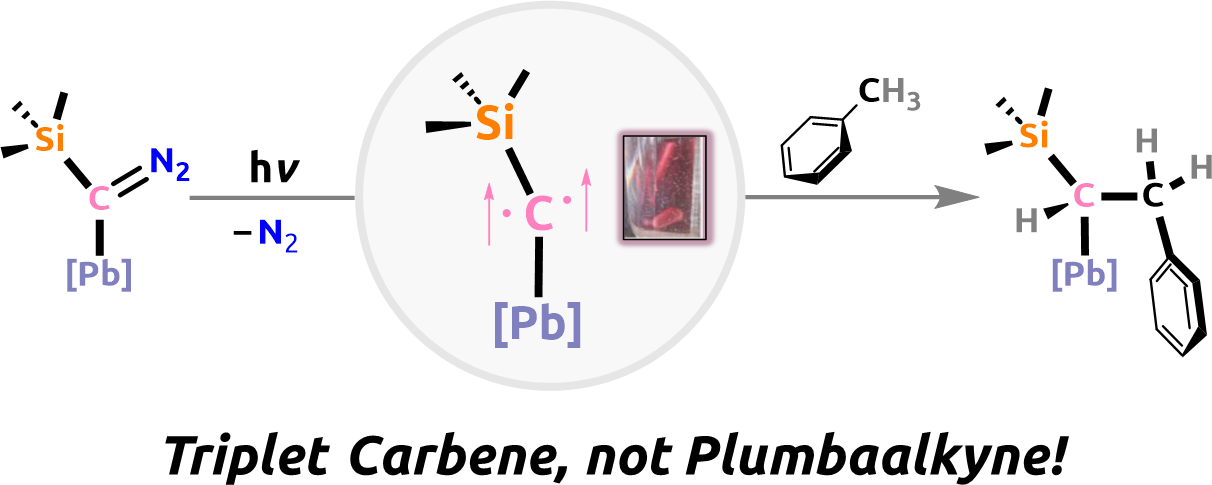
We presented for the first time a persistent (in the cold) triplet carbene stabilized by a Pb(II) substituent. Accordingly, we contributed the first report of a bis-oxidized singlet-carbene together with the Bertrand group. Currently, we are super-excited about bond activation with Lewis super-acidic Phosporus-based radicals and dications.
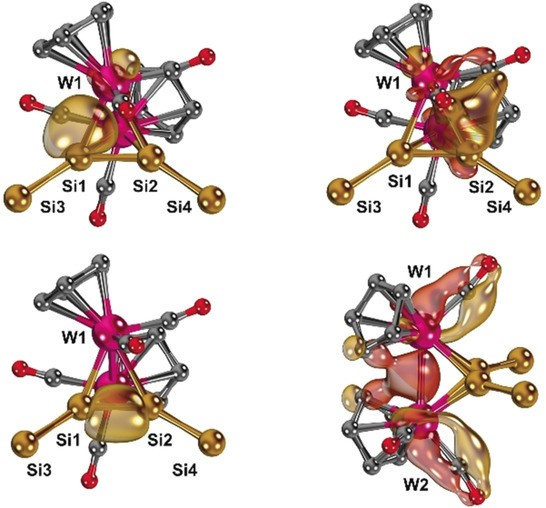
We have been involved in the computational analysis of various molecules with intriguing electronic properties. This includes, among others, an iron(VII) nitride, homoleptic "chiral" uranium complexes, open-shell alkyne complexes of the 3d metals, molybdenum and tungsten silylidynes and their dimers, P4 activation by silyl stannylenes, molybdenum imido complexes, iron cyclopentadienyl complexes in unusual oxidation states, and aluminium(I) species for Fischer-Tropsch chemistry.





































