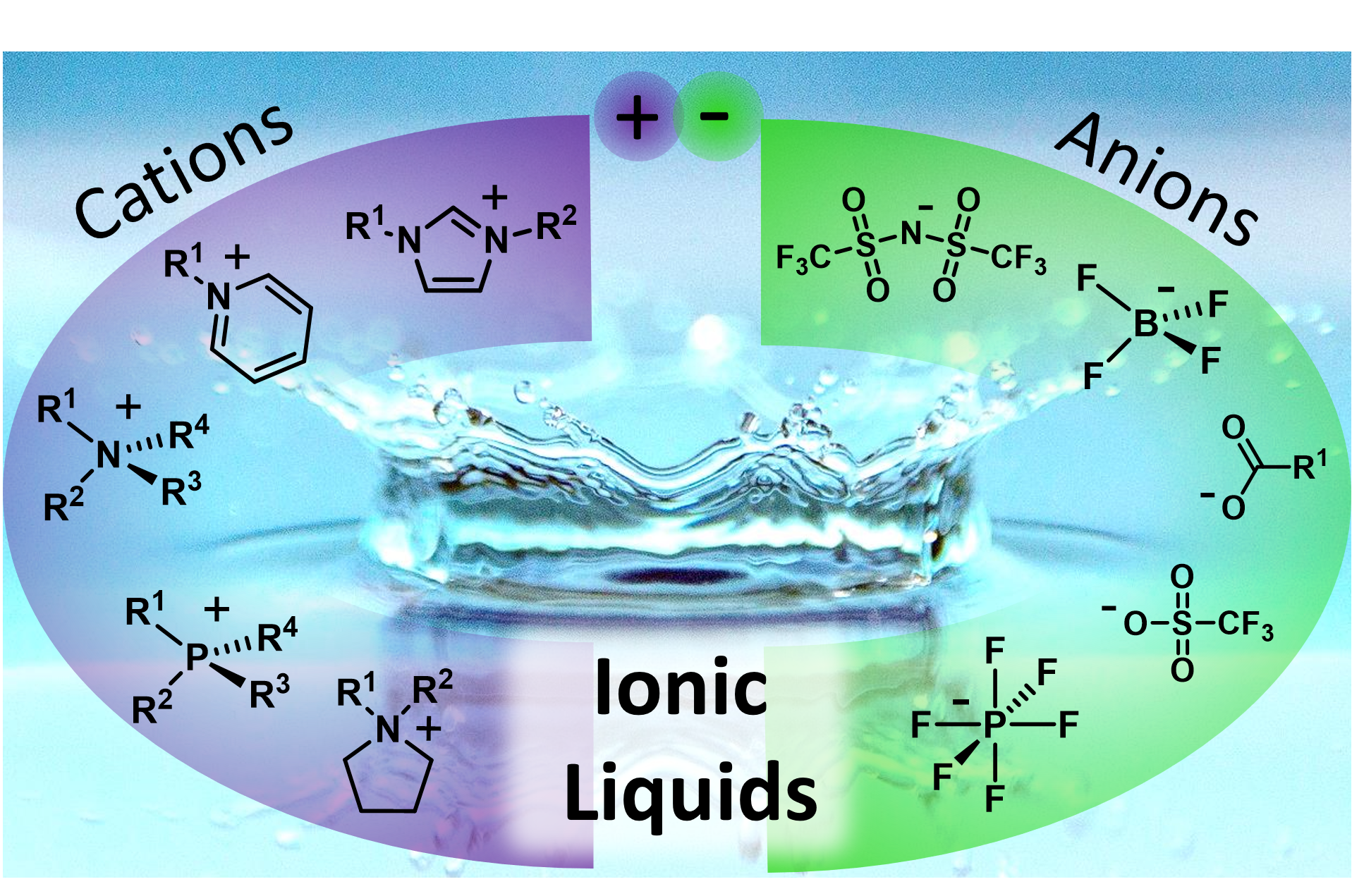
Ionic Liquids
Ionic liquids are organic salts with exceptionally low melting points (commonly below 25°C) forming a fascinating, diverse class of modern high-performance liquid materials. They conflate beneficial properties of molecular liquids and conventional high temperature molten salts to their own unique characteristics. These features include negligible volatility and non-inflammability, intrinsic conductivity as well as high thermal, chemical and electrochemical stability giving overall safe and powerful liquids. Furthermore, ionic liquids offer the exceptional possibility for the tuning of their properties via the molecular structure towards a particular application. As a result of these advanced properties, ionic liquids have found numerous actual and potential applications ranging from niche uses to large-scale industrial processes.
Research
Our application-oriented research covers the two different approaches for designing, synthesizing and optimizing ionic liquids for particular technical implementations – the investigation of structure-property-relationships and the reverse engineering approach for scientific cooperation.
For the first approach we synthesize novel ionic liquids with different cations, anions and functional groups and measure their macroscopic properties in correlation to their microscopic, chemical structure. Our main focus is thereby the maximization of conductivity which is essential for their use in effective and safe electrochemical devices, such as supercapacitors and rechargeable batteries, which are essential for the transition towards a sustainable energy supply. Fundamental investigations are still essential to understand the interrelations of the ionic liquid properties, especially as there is an enormous number of representatives synthetically accessible. Furthermore, there are still many unsolved questions regarding their liquid structures, interrelation of transport properties and interactions with other materials, such as interfaces or polymers, which we try to address with our research.
For the reverse design approach, we are using our in-depth knowledge about the synthesis of ionic liquids and interrelations of the chemical motifs and properties to support our cooperation partners with samples that are suitable for their field of research. The applications to which we contribute are quite diverse and range from physics over applied materials to sustainable technologies. We also try to help our collaborators by synthesizing ionic liquids that help to answer specific scientific questions. Therefore, we also gained familiarity with the preparations of ionic liquids with different deuteration patterns which could be used for instance in NMR spectroscopy and neutron scattering experiments.
We have close and fruitful cooperation with research groups focusing on:
- Battery-technology
- Supercapacitors
- Redox-flow batteries
- Fuel cells
- Tribology
- Utilization of renewable resources (lignin and cellulose)
- Spectroscopy
- Neutron scattering
- Polymer materials
► CV
2009 - 2014: Diploma studies at Karlsruhe Institute of Technologie (KIT) and Johannes Gutenberg University Mainz
2014 - 2019: PhD studies in the group of Prof. Dr. Hempelmann at Saarland University. Working on third party projects of Deutsche Bundesstiftung Umwelt (DBU), Deutsche Forschungsgemeinschaft (DFG) and Arbeitsgemeinschaft industrieller Forschungsvereinigungen (AiF) dealing with the use of ionic liquids in sustainable technologies
Since 2019: PostDoc at Saarland University in the group of Prof. Dr. Kay
Recent Publications
A. Hofmann, D. Rauber, T.-M. Wang, R. Hempelmann, C.W.M. Kay, T. Hanemann; Novel Phosphonium-Based Ionic Liquid Electrolytes for Battery Applications; Molecules,2022, 27, 4729. DOI: https://doi.org/10.3390/molecules27154729.
S. Koutsoukos, F. Philippi, D. Rauber, D. Pugh, C.W.M. Kay, T. Welton; Effect of the cation structure on the properties of homobaric imidazolium ionic liquids; Phys. Chem. Chem. Phys., 2022,24, 6453-6468. (2022 PCCP HOT Articles) DOI: https://doi.org/10.1039/D1CP05169E
F. Philippi, D. Rauber, O. Palumbo, K. Goloviznina, J. McDaniel, D. Pugh, S. Suarez, C.C. Fraenza, A. Padua, C.W.M. Kay, T. Welton;Flexibility is the key to tuning the transport properties of fluorinated imide-based ionic liquid; Chem. Sci., 2022, 13, 9176-9190. (Pick of the Week Collection, HOT Article Collection) DOI: https://doi.org/10.1039/D2SC03074H
F. Philippi, D. Rauber, K.L. Eliasen, N. Bouscharain, K. Niss, C.W.M. Kay, T. Welton; Pressing matter: why are ionic liquids so viscous?; Chem. Sci., 2022, 13, 2735-2743. DOI:https://doi.org/10.1039/D1SC06857A
D. Rauber, F. Philippi, B. Kuttich, J. Becker, T. Kraus, P. Hunt, T. Welton, R. Hempelmann, C.W.M. Kay; Curled cation structures accelerate the dynamics of ionic liquids; Phys. Chem. Chem. Phys., 2021, 23, 21042-21064. DOI: https://doi.org/10.1039/D1CP02889H.
D. Rauber, A. Hofmann, F. Philippi, C.W.M. Kay, T. Zinkevich, T. Hanemann, R. Hempelmann; Structure-Property Relation of Trimethyl Ammonium Ionic Liquids for Battery Applications. Appl. Sci., 2021, 11, 5679. https://doi.org/10.3390/app11125679.
K.L. Eliasen, H.W. Hansen, F. Lundin, D. Rauber, R. Hempelmann, T. Christensen, T. Hecksher, A. Matic, B. Frick, K. Niss; High-frequency dynamics and test of the shoving model for the glass-forming ionic liquid Pyr14-TFSI, Phys. Rev. Materials 5, 2021, 065606. DOI: https://doi.org/10.1103/PhysRevMaterials.5.065606
F. Lundin, H.W. Hansen, K. Adrjanowicz, B. Frick, D. Rauber, R. Hempelmann, O. Shebanova, K. Niss, and A. Matic; Pressure and Temperature Dependence of Local Structure and Dynamics in an Ionic Liquid; J. Phys. Chem. B 2021 125 (10), 2719-2728. https://doi.org/10.1021/acs.jpcb.1c00147
F. Philippi, D. Rauber, B. Kuttich, T. Kraus, C.W.M. Kay, R. Hempelmann, P. A. Hunt, T. Welton; Ether functionalisation, ion conformation and the optimisation of macroscopic properties in ionic liquids; Phys. Chem. Chem. Phys., 2020, 22, 23038-23056. DOI: https://doi.org/10.1039/D0CP03751F
F. Philippi, D. Pugh, D. Rauber, T. Welton, P.A. Hunt; Conformational design concepts for anions in ionic liquids; Chem. Sci., 2020, 11, 6405-6422. DOI: https://doi.org/10.1039/D0SC01379J